How Do You Know How Many Steps a Reaction Occurs in
| ORDERS OF REACTION AND MECHANISMS This page looks at the relationship between orders of reaction and mechanisms in some uncomplicated cases. It explores what a mechanism is, and the idea of a rate determining step. It too explains the difference between the sometimes confusing terms "lodge of reaction" and "molecularity of reaction". | |
| Note:If you aren't sure nigh orders of reaction you ought to read the introductory page before you get on. You will find a link back to here at the lesser of the introductory page. | |
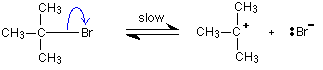
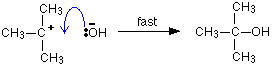
| Reaction mechanisms What is a reaction mechanism? In whatever chemical change, some bonds are cleaved and new ones are made. Quite often, these changes are too complicated to happen in one simple stage. Instead, the reaction may involve a serial of small changes one after the other. A reaction mechanism describes the one or more steps involved in the reaction in a mode which makes information technology clear exactly how the diverse bonds are broken and made. The post-obit example comes from organic chemical science. Information technology doesn't thing in the least if it is unfamiliar to yous! This is a reaction betwixt ii-bromo-ii-methylpropane and the hydroxide ions from sodium hydroxide solution: The overall reaction replaces the bromine atom in the organic chemical compound by an OH group. The first thing that happens is that the carbon-bromine bond in a small proportion of the organic compound breaks to requite ions: Carbon-bromine bonds are reasonably strong, so this is a irksome change. If the ions hit each other once more, the covalent bond will reform. The curly arrow in the equation shows the movement of a pair of electrons. If there is a high concentration of hydroxide ions present, the positive ion stands a high adventure of hitting ane of those. This stride of the overall reaction will exist very fast. A new covalent bond is fabricated between the carbon and the oxygen, using one of the lone pairs on the oxygen atom. Considering carbon-oxygen bonds are strong, in one case the OH grouping has attached to the carbon atom, it tends to stay attached. The mechanism shows that the reaction takes place in two steps and describes exactly how those steps happen in terms of bonds being broken or fabricated. It also shows that the steps have different rates of reaction - one slow and one fast. | |
| Annotation:If you are interested in exploring more organic reaction mechanisms you will find probably more than you will want to know about past following this link! | |
| The charge per unit determining stride The overall charge per unit of a reaction (the ane which you would measure if you did some experiments) is controlled by the rate of the slowest stride. In the example above, the hydroxide ion can't combine with the positive ion until that positive ion has been formed. The second footstep is in a sense waiting around for the get-go slow step to happen. The slow stride of a reaction is known equally the rate determining pace . Equally long every bit there is a lot of difference between the rates of the various steps, when you measure the rate of a reaction, yous are actually measuring the rate of the charge per unit determining stride. Reaction mechanisms and orders of reaction The examples we use at this level are the very simple ones where the orders of reaction with respect to the various substances taking role are 0, 1 or two. These tend to have the irksome step of the reaction happening before any fast step(s). To endeavor to explain how fractional orders of reaction tin arise is beyond the scope of UK A' level courses. Example 1 Here is the mechanism we have already looked at. How do we know that it works similar this? By doing rate of reaction experiments, yous find this rate equation: The reaction is first social club with respect to the organic compound, and nil gild with respect to the hydroxide ions. The concentration of the hydroxide ions isn't affecting the overall charge per unit of the reaction. If the hydroxide ions were taking part in the tiresome pace of the reaction, increasing their concentration would speed the reaction upward. Since their concentration doesn't seem to thing, they must be taking part in a later fast step. Increasing the concentration of the hydroxide ions will speed up the fast step, but that won't have a noticeable upshot on the overall charge per unit of the reaction. That is governed by the speed of the slow pace. | |
| Note:If you decreased the concentration of hydroxide ions plenty, you will eventually wearisome downward the 2d step of this reaction to the point where both steps have similar rates. At that betoken, the concentration of the hydroxide ions will matter. At normal concentrations, the rates of the 2 steps differ widely, and so this problem doesn't ascend. | |
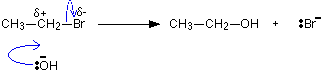
| In a unproblematic case similar this, where the slow step of the reaction is the first stride, the rate equation tells you what is taking part in that slow pace. In this example, the reaction is beginning guild with respect to the organic molecule - and that's all. This gives you a starting indicate for working out a possible machinery. Having come up upwards with a mechanism, you would need to detect more show to confirm it. For instance, in this instance you might endeavour to notice the presence of the positive ion that is formed in the first step. Case 2 At first sight this reaction seems identical with the last 1. A bromine cantlet is beingness replaced by an OH grouping in an organic compound. Even so, the rate equation for this plain similar reaction turns out to be quite unlike. That means that the mechanism must be different. The reaction this fourth dimension is first order with respect to both the organic compound and the hydroxide ions. Both of these must be taking function in the wearisome footstep of the reaction. The reaction must happen by a straightforward collision between them. The carbon atom which is hit by the hydroxide ion has a slight positive accuse on information technology and the bromine a slight negative ane considering of the difference in their electronegativities. As the hydroxide ion approaches, the bromine is pushed off in ane smooth action. | |
| Note:If you are interested in understanding these mechanisms in more than item, y'all could follow this link. For the purposes of this page, all that matters is that the rate equations evidence that the ii plainly similar reactions happen by different mechanisms. | |
| Molecularity of a reaction You may come up across an older term known equally the molecularity of a reaction. This has largely dropped out of UK A' level syllabuses, just if you meet information technology, information technology is important that you understand the difference between this and the gild of a reaction. The terms were sometimes used carelessly as if they mean the aforementioned thing - they don't! Gild of reaction The important matter to realise is that this is something which can just be found by doing experiments. It gives you information well-nigh which concentrations affect the rate of the reaction. You cannot await at an equation for a reaction and deduce what the order of the reaction is going to exist - you have to practise some applied work! Having plant the lodge of the reaction experimentally, y'all may be able to make suggestions most the mechanism for the reaction - at least in unproblematic cases. Molecularity of a reaction This starts at the other stop! If you know the mechanism for a reaction, y'all can write downwardly equations for a serial of steps which make information technology up. Each of those steps has a molecularity. The molecularity of a stride simply counts the number of species (molecules, ions, atoms or costless radicals) taking office in that step. For case, going dorsum to the mechanisms we've been looking at: This step involves a single molecule breaking into ions. Considering only 1 species is involved in the reaction, it has a molecularity of one. It could be described as unimolecular. The 2nd stride of this machinery, involves two ions reacting together. This step has a molecularity of ii - a bimolecular reaction. The other reaction we looked at happened in a single step: Because of the two species involved (ane molecule and one ion), this reaction is also bimolecular. Unless an overall reaction happens in one footstep (like this last one), you can't assign information technology a molecularity. You have to know the mechanism, and then each private step has its own molecularity. There's nothing the least bit complicated about the term molecularity. The only defoliation is that you may sometimes notice it used every bit if it meant the same every bit gild. Information technology doesn't! More about reaction mechanisms and orders of reaction Relating orders of reaction to mechanisms is relatively piece of cake where the dull stride is the first step of the reaction mechanism. It isn't so easy when it is one of the later on steps. I want to look at this in a flake more detail in instance your syllabus requires it. Nosotros volition revisit the uncomplicated case starting time where the boring stride is the first step of the mechanism. Cases where the slow step is the first step in the machinery Suppose you had a reaction betwixt A and B, and it turned out (from doing some experiments) to be first order with respect to both A and B. Then the rate equation is:
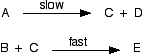
Which of these ii mechanisms is consistent with this experimental finding? Mechanism i Mechanism 2 Remember that in uncomplicated cases, where the slow step is the commencement step of the mechanism, the orders tell you what is taking office in the slow step. In this example, the reaction is first society with respect to both A and B, so i molecule of each must be taking part in the slow footstep. That means that mechanism 2 is possible. However, mechanism 1 must be wrong. One molecule of A is taking part in the tiresome stride, but no B. The rate equation for that would be:
| |
| Note:Be conscientious! You can't be certain that machinery 2 is correct - it may be, but you can't be sure. A and B could, for example, react to give some sort of intermediate, which went on to turn into D and E by ane or more fast steps. The charge per unit equation would be the same, because information technology is governed by the same boring footstep, but y'all tin't be certain about what happens after that. All you tin be sure of is that machinery 1 is inconsistent with the charge per unit equation, and so is wrong. | |
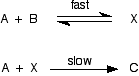
| Cases where the slow pace isn't the outset step in the mechanism This is much more difficult to practice and explain. I'm going to start with equally simple example as possible Example 1 Suppose the mechanism for a reaction betwixt A and B looks like this: This time the slow step is the second step. Discover that the kickoff (fast) pace is reversible. I need to presume that the fast pace is much faster than the deadening step - for reasons that I'm not going to explain. We are looking well across A level here! The rate of the reaction will be governed past the slow step, so the rate equation might look like this:
. . . except, of course, that X isn't one of the things y'all are starting with! At an introductory level, the flawed discussion frequently goes something like this:
As it happens, that gives the right answer in this instance, only this simplistic view doesn't work in all cases - as I will show below. So let's start again and do it improve! The charge per unit of the reaction will be governed by the slow step, then the rate equation might look similar this:
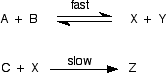
. . . except, of form, that Ten isn't one of the things you are starting with! You lot need to be able to express the concentration of Ten in terms of [A] and [B], and you lot can do that because the get-go pace is an equilibrium. The equilibrium abiding for the beginning reaction is: Y'all can rearrange this to give an expression for [X]: . . . and and so substitute this value into the rate expression nosotros started with: If you lot sort this out, and combine the ii different constants into a new rate constant, you get the charge per unit expression: So the reaction is 2nd order with respect to A and first society with respect to B. Example 2 I am going to take another like-looking example now, chosen to exist deliberately bad-mannered. This is to try to bear witness that y'all tin't reliably work these problems out just past looking at them. This relates to the following reaction: The mechanism for this is: From the slow step of the reaction, the rate equation would like similar this:
Upwardly to now, this looks but the same as the previous example - merely it isn't! Let's exercise it properly, and think nigh the equilibrium expression for the first step in club to find a value for [X] that nosotros can substitute into the rate equation. The problem is that we have now got an actress variable in this equation that wasn't there earlier - nosotros have got the concentration of Y. What practise we practise nearly that? Well, for every mole of X formed, a mole of Y will exist formed besides. Provided that at that place was no X or Y in the mixture to first with, that ways that the concentration of Y is equal to the concentration of X, and so nosotros can substitute a value of [X] in place of the [Y], giving: Rearranging that to get an expression for [10] gives: And now yous tin substitute this into the rate equation: . . . and then tidy it up by combining the two constants into a unmarried new charge per unit constant: The guild is 0.v with respect to both A and B, and 1 with respect to C. At that place is no mode you could have come up with that answer if you had just looked at the equations and assumed that the concentration of Ten is proportional to the concentrations of A and B. The presence of the extra Y makes a serious deviation to the result - simply just looking at the equations, that isn't at all obvious. | |
| Notation:Syllabuses often aren't at all clear about what the examiners actually desire with regards to this. You have to look at by papers and marker schemes. Of the United kingdom-based exams, I know that CIE have asked a question relating to a machinery where the slow step was either the second or tertiary in a series of iii steps. But they were simply expecting students to only look at the equations, and not work it out properly. Personally, I think this is misleading and wrong. | |
To the rates of reaction menu . . . To the Concrete Chemistry carte du jour . . . To Principal Menu . . . © Jim Clark 2002 (final modified October 2013) | |
harperwhisingerrim.blogspot.com
Source: https://www.chemguide.co.uk/physical/basicrates/ordermech.html
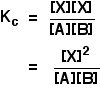
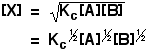
0 Response to "How Do You Know How Many Steps a Reaction Occurs in"
Post a Comment